Ressources

All our news
ITCC-P4, first company in the world to offer preclinical drug tests for children with cancer

Publications
Preclinical In Vivo Validation of the RAD51 Test for Identification of Homologous Recombination-Deficient Tumors and Patient Stratification
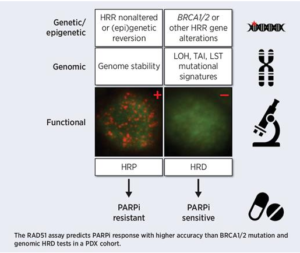
Publications
Targeting genome integrity dysfunctions impedes metastatic potency in non-small-cell lung cancer circulating tumor cellderived eXplants
CTC-derived eXplants (CDX) offer systems for mechanistic investigation of CTC metastatic potency and may provide rationale for biology-driven therapeutics.
Publications
STING protects breast cancer cells from intrinsic and genotoxic-induced DNA instability via a non-canonical, cell-autonomous pathway – Oncogene, Oct.8 2021
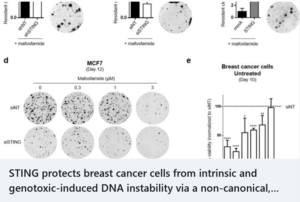
news
ITCC-P4, first company in the world to offer preclinical drug tests for children with cancer

news
XenTech remains fully operational after 7 weeks of COVID-19 pandemic : update from our COO
In this 7th week of containment and as end of confinement in France is announced for May 11th, I would first of all like to thank you on behalf of our teams for all the messages of support you have sent us since the beginning of the COVID-19 crisis.
news
XenTech Covid-19 management
Regarding the current circumstances, we at XenTech want you to know that we are taking the Covid-19 pandemia very seriously. We are closely monitoring the pandemic, following guidance and advice from the World Health Organization (WHO), and the French Government.
publications
Preclinical In Vivo Validation of the RAD51 Test for Identification of Homologous Recombination-Deficient Tumors and Patient Stratification

publications
Targeting genome integrity dysfunctions impedes metastatic potency in non-small-cell lung cancer circulating tumor cellderived eXplants
CTC-derived eXplants (CDX) offer systems for mechanistic investigation of CTC metastatic potency and may provide rationale for biology-driven therapeutics.
publications
STING protects breast cancer cells from intrinsic and genotoxic-induced DNA instability via a non-canonical, cell-autonomous pathway – Oncogene, Oct.8 2021

publications
Paediatric Liver Cancer
A preclinical platform of mouse-transplanted human pediatric liver tumors to evaluate the efficacy of conventional and innovative anticancer therapy
01Conferences & events
04.25.25
We’re excited to share our upcoming presentations of two posters at the American Association for Cancer Research (AACR) Annual Meeting 2025, happening April 25-30 in Chicago, IL!
 Xentech is willing to accelerate the preclinical development/validation of new anti-cancer therapeutics with a growing panel of highly predictive human tumor-based models and robust screening/profiling studies.
Looking forward to welcoming you at booth #538 or in front of the posters !
Xentech is willing to accelerate the preclinical development/validation of new anti-cancer therapeutics with a growing panel of highly predictive human tumor-based models and robust screening/profiling studies.
Looking forward to welcoming you at booth #538 or in front of the posters !
10.23.24
XenTech exhibits at EORTC-NCI-AACR (Barcelona, Oct. 23-25 2024)
 We'll be exhibiting at EORTC-NCI-AACR in Barcelona, stop by booth #17 !
We'll be exhibiting at EORTC-NCI-AACR in Barcelona, stop by booth #17 !
04.05.24
XenTech will be attending AACR Annual Meeting in San Diego (Apr. 5-10 2024)
 Olivier, Vincent and Marie cordially invite you to stop by our Booth#3645 to discuss your innovative research projects.
Olivier, Vincent and Marie cordially invite you to stop by our Booth#3645 to discuss your innovative research projects.
04.14.23
XenTech will attend AACR Annual Meeting in Orlando (Apr. 14-19 2023)
 Philippe, Olivier and Delphine cordially invite you to stop by our Booth#618 to discuss our solid tumor PDX collection to bring your innovative research projects a step further !
Philippe, Olivier and Delphine cordially invite you to stop by our Booth#618 to discuss our solid tumor PDX collection to bring your innovative research projects a step further !



